

Our goal is to accelerate the translation of novel diagnostics into real, accessible benefits for patients

Ensure that participant care and safety is at the heart of all we do.
Care and Safety
01

Our Values
02
Rigour and Integrity
Adhere to the highest standards of scientific and ethical conduct.
03
Collaboration
Work with the world's experts across industry and academia to deliver results.
About Nutromics
Nutromics exists to save millions of lives through Continuous Diagnostic Monitoring
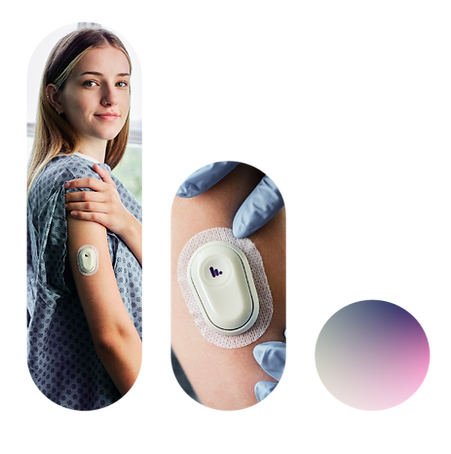
Clinicians today are hampered by insufficient and delayed lab diagnostics. Typically, a clinician orders a test for a patient, a blood draw is taken, the sample is then sent to the lab where it’s processed, and finally, the clinician receives a single data point of where the patient was hours prior. This standard of care is not equipped to provide trend information, which is critical in the ICU for fast-moving disease states and dynamic patient conditions. The result is poor patient outcomes and billions in additional healthcare costs running a highly inefficient system. Our diagnostic platform is like a lab-on-a-patch. It is a breakthrough technology combining multiple DNA-based sensors with minimally invasive microneedles. Our powerful technology can track multiple crucial targets continuously and in real-time, giving clinicians critical, personalized insights.

The Human Research Ethics Committee (HREC)

Nutromics Diagnostics HREC's mission is to provide Australian researchers with diagnostic-specific expertise and focus
The ND-HREC functions to: 1. Provide independent oversight of non-interventional diagnostic research in humans, and clinical studies that support the development of diagnostic tools or methods. 2. Provide competent, timely review and monitoring of human research in respect of their ethical and scientific acceptability for as long as research is active; 3. Determine the compliance of a human research with the National Statement and grant, withhold or withdraw ethical approval.
Clinical Trials

Limit of Blank Characterization of Vancomycin Biosensor Nutromics Device
Study Design
This is a prospective, non-dosing, interventional study to determine the response of the Nutromics Vancomycin Biosensor Devices on healthy humans, subjected to different conditions and electrode configurations.
Planned Sample Size
Up to 50 participants
Status
Currently enrolling

VANCORE: VANcomycin dosing study to CORrElate between compartments: a pilot characterisation study of a new device.
Study Design
This is a prospective, open-label, single-cohort dosing study to establish the correlation of the Area Under the Curve (AUC) of vancomycin between serum and interstitial fluid (ISF). Participants will receive 4 doses of vancomycin at varying concentrations on separate occasions.
Planned Sample Size
12 healthy participants
Status
Currently enrolling

Register your interest
Register your interest by reaching out with your name and contact details to: clinical.research@nutromics.com

Frequently Asked Questions (FAQs)

Follow
Location
Nutromics Clinical Research Centre
420 Victoria Street, Brunswick 3056
VIC Australia